Direct Analysis in Real-Time Mass Spectrometry (DART-MS) revolutionizes chemical analysis by rapidly detecting flame retardants, phthalates, and polyaromatic amines without extensive sample preparation. In this blog post, we explore the technique’s advantages, delve into its process, and highlight its applications.

Direct Analysis in Real Time Mass Spectrometry (DART-MS) is a technique used in analytical chemistry combining two methods: Direct Analysis in Real Time (DART) and Mass Spectrometry (MS). It uses DART as the ionization source for mass spectrometry analysis. A stream of energetic ions strikes the sample, causing the molecules to become ionized. The mass spectrometer then separates these ions based on their mass-to-charge ratio (m/z) and detects them. This process allows for compound identification and quantification in the sample.
It involves the following steps:
The processes differ depending on the type of ionization source system. There are two popular ionization source systems, both of which use helium for operation:
1. DART/IonRocket (Pyrolysis Option)
This method is relatively simple regarding sample preparation but may not yield quantitative results.
The IonRocket component introduces a pyrolysis option involving the controlled thermal decomposition of a sample in the absence of oxygen. This method is beneficial for analyzing and pre-screening polymers, complex materials, or samples requiring controlled thermal decomposition before ionization.
To introduce liquid or solid samples, they are placed in a small metallic vessel below the DART system and heated directly in a controlled manner. After heating, the pyrolyzer vaporizes the samples and, in the next step, ionizes using the DART source before being introduced to mass spectrometry.
2. DART/QuickStrip:
The DART/QuickStrip configuration incorporates the QuickStrip Sample Introduction System into the DART ionization source. This automated DART method utilizes a Solid Phase Mesh Enhanced Sorption from Headspace (SPMESH) with 12 spots for samples.
It requires extensive sample preparation, including grinding and extraction. For example, based on IEC 62321, the lab technician must dissolve samples in the proper solution and finish the process with Soxhlet extraction. Once extracted, the SPMESH can take a drop of each sample for analysis.
While this method uses less helium than the IonRocket, it offers the option to add an internal standard for more accurate qualitative results. The analysis starts when the SPMESH is located on the rail in front of the ion source, and each sample takes a few seconds to complete.
Then, ionize the sample by exposing it to the DART source.
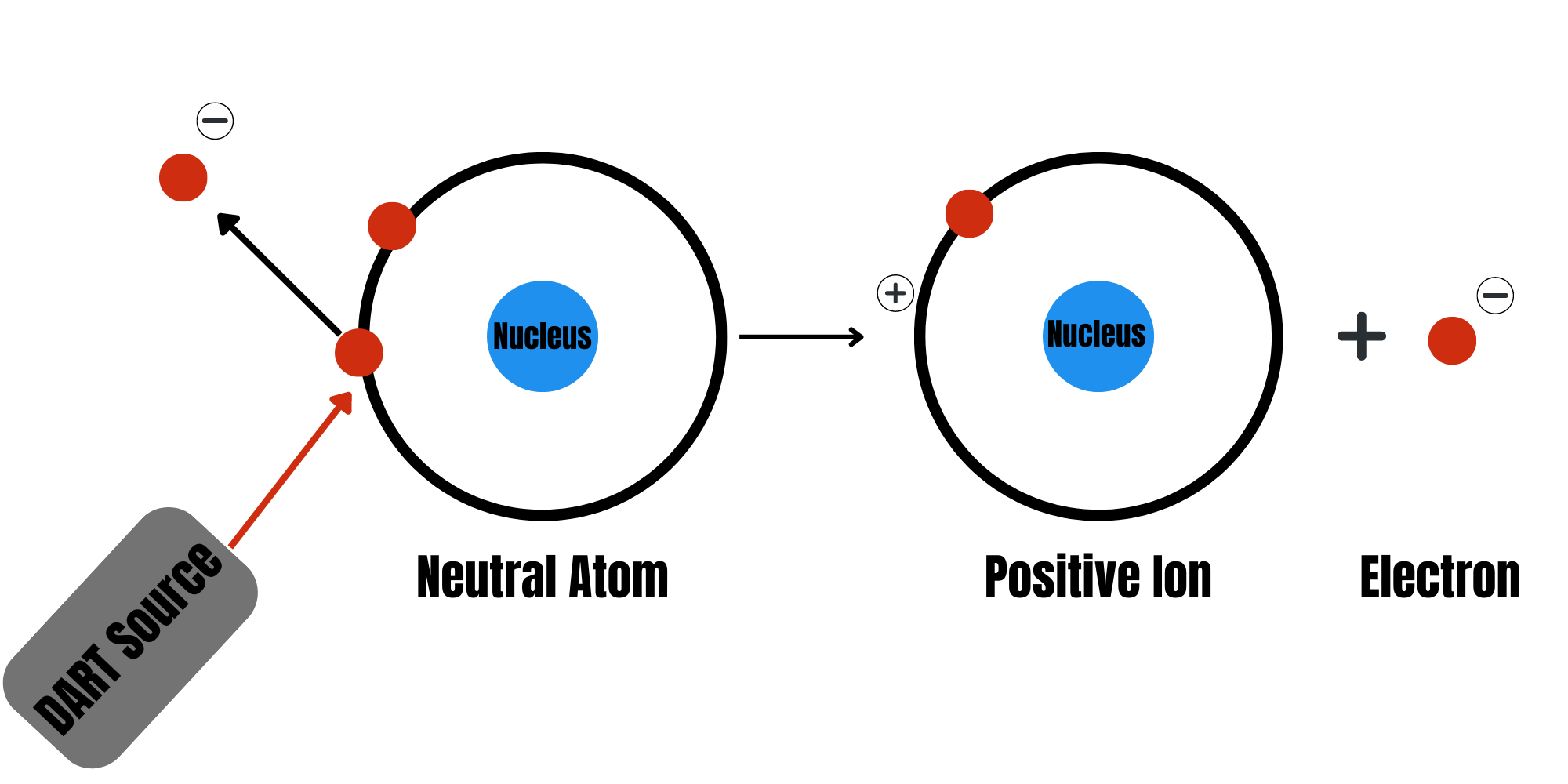
What is Ionization?
Ionization is the process of adding or removing electrons from an atom or neutral molecule, resulting in a net electric charge. Mass spectrometry (MS) analysis relies on the mass-to-charge ratio (m/z) for operation.
How Does Ionization Occur in DART-MS Analysis?
In this type of analysis, the DART source produces a high-temperature stream of plasma composed of ions and electrons. This plasma stream ionizes the molecules present in a sample in an ambient atmosphere without the need for prior separation or chromatographic techniques. Additionally, the molecules present within the samples can become ionized in many ways, such as:
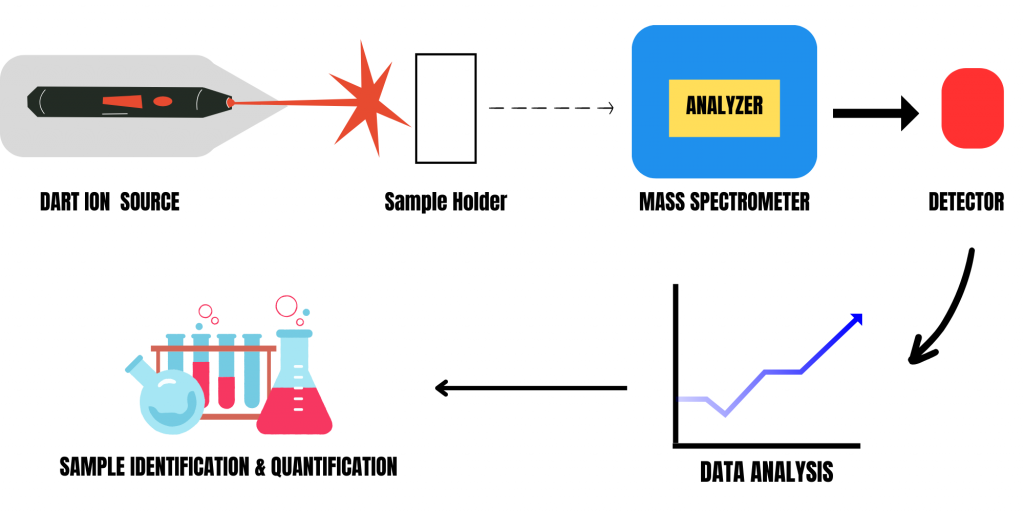
Subsequently, ions generated in the DART ionization source are analyzed using mass spectrometry. Inside a mass spectrometer, the analyzer separates ions by their m/z. Common examples of analyzers used in MS include:
The detector receives the separated ions one after the other, generating an electrical signal proportional to their abundance. It’s important to note that DART is incompatible with the MS system of gas chromatography-mass spectrometry (GC-MS) instruments. However, most DART systems are compatible with the MS system of liquid chromatography-mass spectrometry (LC-MS). As mentioned earlier, TOF mass analyzers can also work with DART. TOF mass analyzers measure the time ions take to travel from the ion source to the detector, providing information to determine the ions’ mass-to-charge ratio.
Various combinations are possible with DART, including:
Moreover, the detector converts the electrical signal into digital data, forming a mass spectrum. This plot represents ion quantity (y-axis) versus m/z (x-axis), enabling the identification and quantification of target compounds present in the sample.
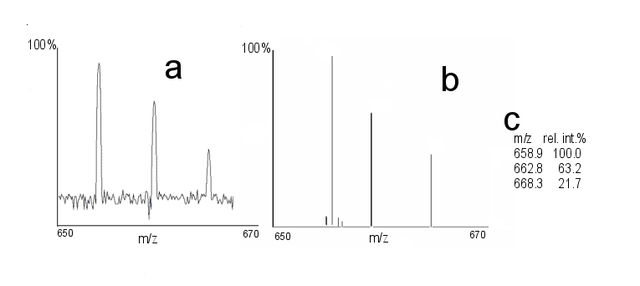
Compound Identification using DART-MS
The obtained spectra are compared to known databases or reference spectra to identify molecules within a sample.
Compound Quantification
Researchers can use the ion abundance striking the detector can be used to estimate the concentration of molecules within a sample.
It offers several distinct advantages that have made it an invaluable tool in chemistry testing:
Unlike traditional analytical methods that involve time-consuming sample preparation, DART-MS provides near-instantaneous analysis. This aspect enables quick on-site testing, benefiting enforcement authorities present in time-sensitive investigations, environmental compliance, and quality control measures.
The instrument operates under ambient conditions eliminating the need for a vacuum or additional heating equipment. Samples can be analyzed in their natural environment in their native form, whether in a liquid, solid, or gaseous state.
This instrument combines ionization principles and mass spectrometry to offer sensitive and accurate results. Indeed, it can detect target compounds such as polymers, making it a great fit for pre-screening samples in a short time, where complicated structures can be challenging to detect using other instruments such as FTIR.
DART-MS allows for using a minimal amount of samples, preserving some for further experimentation. This advantage maximizes sample utility compared to other analytical techniques.
Additionally, one of the advantages of DART-MS is its ability to analyze samples without the need for extensive preparation depending on ionization source system. Some MS techniques require complex extraction, purification, or derivatization steps. However, a benefit of DART-MS/IonRocket is that it can analyze samples in their original form. This convenience simplifies the workflow and reduces the potential for errors or contamination, enhancing efficiency and productivity.
This method can analyze a variety of sample types including:

The minimal sample preparation and rapid analysis allow a relatively low-cost technique. Therefore, enforcement authorities tend to use DART-MS to verify the environmental compliance of products. However, please remember that a DART system costs two to four times a GC/MS cost.
Just like any chemical analysis technique, that one presents some disadvantages, including:
DART-MS may have lower ionization efficiency compared to some other ionization techniques. Thus, improving an analyte’s ionization efficiency and sensitivity may require specific sample treatments.
Matrix effects can affect this technique. Co-eluting compounds or matrix components can cause ion suppression or enhancement effects. This phenomenon may impact the accuracy of quantitative results. Therefore, to improve selectivity, one may require additional separation techniques.
What is a Matrix?
In the context of DART-MS, a matrix is a substance mixed with an analyte. It can:
Compared with other fragmentation-type techniques, it may be challenging to decipher complex molecular structures with DART-MS. Indeed, limited fragmentation patterns characterize this technique. Therefore, comprehensive structural analysis may require complementary methods.
DART-MS finds applications in various industries where rapid and accurate analysis is crucial. For environmental analysis, it can detect and pre-screen the presence of pollutants such as:
DART-MS detects whether a product contains Persistent Organic Pollutants (POPs) in a product, making it useful for environmental compliance purposes as a pre-screening technique. For example, DART-MS identifies POPs like Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs).
Moreover, Restriction of Hazardous Substances (RoHS) regulations cover some POPs like PBBS. Therefore, using DART-MS to establish their presence aids in the compliance process.

This technique rapidly screens and identifies VOC pollutants in the air, water, soil, and petrochemical samples. VOCs to watch out for include:
DART-MS swiftly detects and pre-screens the presence of flame retardants ensuring compliance with safety regulations for textile, electronics, and construction materials.
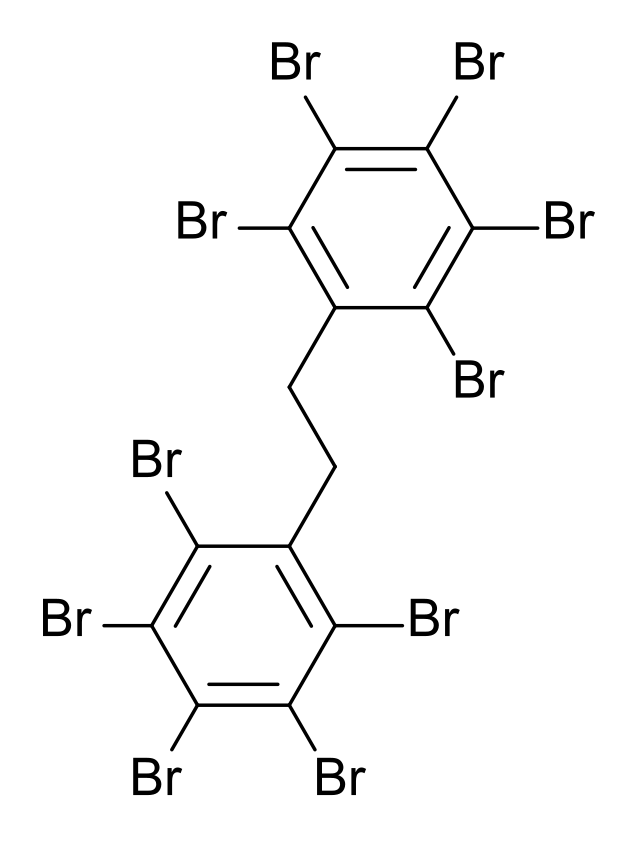
Moreover, DART-MS plays a vital role in identifying and pre-screening phthalates in consumer products, such as toys, cosmetics, and food packaging. Regulatory authorities can use this information to enforce safety standards and protect consumers.
DART-MS plays an instrumental role in detecting polyaromatic amines. These molecules are carcinogens in plastics, pesticides, tobacco smoke, and environmental samples.

Finally, opt to operate this instrument by trained personnel. Executing tests by an ISO 17025-certified laboratory ensures efficient sample analysis and reliable results.
Any questions? Request your free consultation with Enviropass